| Background |
 |
Our laboratory, supervised by Dr Chang Chan Fong, is mainly involved in
signal transduction work which will include basic molecular biology techniques
as well as using standard biochemical techniques tools. Bioinformatics and biocomputing
will play an integral part in our project work if implemented in our laboratory. We
can rely on online databases and biocomputing tools for experimental design
as well as data analysis. One area of research is in the signaling pathway of the
ectoenzyme, CD38.
In my Honour's Project, I have
characterized and expressed the CD38-GFP protein in mammalian cells.
| What
is CD38? |
 |
CD38
is a type II transmembrane glycoprotein of 300 amino acids expressed in many
vertebrate cells. The deduced amino acid sequences of CD38 have shown that the
polypeptide consists of three domains, a short NH2-terminal
cytoplasmic tail, a single transmembrane region, and a large extracellular COOH-terminal
catalytic domain. It is a bifunctional ectoenzyme that catalyzes both the
synthesis of cyclic ADP-ribose (cADPR) from NAD+ and the degradation
of cADPR to ADP-ribose by means of its ADP-ribosyl cyclase and cADPR-hydrolase
activities, respectively.
(Please
place your mouse on the various compounds to see their descriptions.)
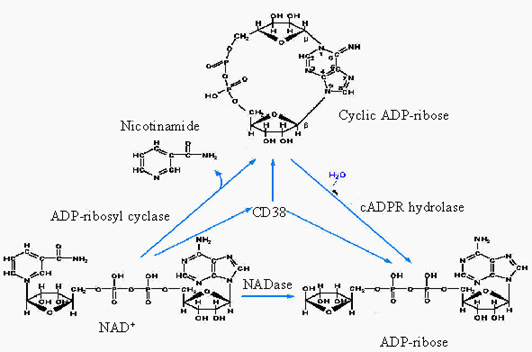
| Roles of CD38 and cADPR |
 |
ADP-
ribosyl cyclase and CD38 are multi-functional enzymes involved in Ca2+ signaling.
Both can cyclize NAD+ and its guanine analog, NGD+, to
produce cyclic ADP-ribose and cyclic GDP-ribose, respectively. Both enzymes can
also catalyze the exchange of nicotinamide group NADP+ with nicotinic
acid (NA) at acidic pH, producing another potent activator of Ca2+
mobilization, nicotinic acid adenine dinucleotide phosphate (NAADP). The Ca2+
release mechanism activated by NAADP is totally independent of cADPR and
inositol triphosphate (IP3).
CD38
plays a role in the signaling system of insulin secretion. Glucose induces an
increase in intracellular Ca2+ concentration in pancreatic b-cells of the islets of Langerhans to secrete insulin.
Recently, CD38 has been demonstrated to be a catalytically
active, unidirectional transmembrane reporter of cADPR, which then reaches its
receptor–operated intracellular calcium stores. Moreover, CD38 was reported to
undergo a selective and extensive internalization through non clathrin-coated
endocytotic vesicles upon incubating CD38+ cells with either NAD+
or thiol compounds: these endocytotic vesicles can convert cytosolic NAD+
into cADPR.
cADPR
is a universal second messenger that releases calcium from intracellular stores
(Clapper et al.,1987).In cells, Ca2+ -induced- Ca2+
-release (CICR) mechanism requires calmodulin and Ca2+ . Ca2+
release is also induced by the CICR modulators, ryanodine and caffeine.Another
well-known mechanism for mobilizing Ca2+ is mediated by inositol
1,4,5-trisphosphate (IP3). Binding of external ligands to
surface receptors can activate phospholipase C and the production of IP3,
which, upon binding to a specific receptor (IP3-sensitive Ca2+
channel) on the endoplasmic reticulum, activates Ca2+ release.
| Approach
used in studying CD38 |
 |
The conventional methodology used in the study of CD38 has always been
immunostaining. GFP
was chosen because its fluorescence is an intrinsic property of the protein,
which does not require additional gene products. Besides, time and effort can be
saved in raising antibodies to localize CD38 in live cells. This
model system enables one to monitor the trafficking and internalization of
receptors in living cells in real time under confocal microscopy.
| Other research
interests in
the laboratory |
 |
The other research
interest in our laboratory is on BST-1 also known as CD157. cADP-ribose acts as
an agent potent in mobilizing Ca2+ from ryanodine-sensitive,
Ins(1,4,5)P3-independent intracellular stores. cADP-ribose is
thought to be an endogenous modulator of ryanodine receptors. Biological
functions of cADP-ribose have been studied in sea-urchin eggs, as well as in a
variety of mammalian cell systems. Convincing evidence is accumulating that cADP-ribose
is involved in cardiac muscle contraction, glucose-induced release of insulin in
endocrine pancreas and contraction of intestinal longitudinal muscle. In
mammalian cells, cADP-ribose is synthesized by the membrane-bound enzymes CD38
and BST-1 /BP-3 , which is now renamed CD157 . Human BST-1/CD157, which was
identified in a bone marrow stromal cell line, consists of 290 amino acids. The
amino acid sequence corresponding to BST-1 cDNA contains an N-terminal signal
sequence and a short C-terminal hydrophobic region, suggesting that BST-1 is a
glycosylphosphatidylinositol (GPI)-anchored membrane protein. Thus BST-1 and
CD38 are bifunctional ecto-enzymes, having both ADP-ribosyl cyclase and cADP-ribose
hydrolase activities. However, both products, cADP-ribose and ADP-ribose, do not
appear to cross the plasma membrane themselves. How cADP-ribose, which is
presumably produced by extracellularly facing ADP-ribosyl cyclase, enters into
the cell interior is currently under investigation.
|

